The Paradox Of A Consistent Need For Variation
By: Ronald L. Shimek Ph.D.
Changes
According to legends and nursery rhymes, King Canute is reputed to have had his throne placed on the beach so he could sit and command the tide not to come in. His command went unheeded, of course, and he and his throne began to get wet. Legend has morphed the tale through the years, though. Old Canute was not trying to stop the tide from coming in; rather he wanted to demonstrate to his over-demanding subjects that even the king was not omnipotent. As time the centuries passed, people forgot what the big picture was and they missed his point. Reef aquarists are seemingly more powerful than Canute; at least in their little puddles they can stop the tides by keeping the water level constant. Perhaps the degree of one’s omnipotence is dependent upon how big a fish one is relative to the size of one’s body of water. On the other hand, however, it may not be in our best interest to mess too much with Mother Nature’s various rhythms.
As I wrote in my previous article, no reef environment is consistent or constant in any factor, except perhaps in that of change. Such variability is not unique to reefs; the shallow water marine environment is, in fact, characterized by change no matter where or when you examine it. The three major reasons for the changes that are a part of the life of all reef organisms are the irradiation and gravitational pull of the sun, leveled with the gravitational pull of the moon and nicely spiced by the inclination of the Earth’s axis. Combined together these three factors generate the tidal, seasonal and climatic variability that characterizes all shallow water marine environments, including those on reefs.
Given that we encounter the effects of climatic variability literally from moment to moment, it seems absurd in the extreme to continually encounter the patently false idea that reef animals must be kept in an environment characterized by constancy in physical factors. Not only do things vary on a reef, the range of variation varies from place to place on a given reef and also between reefs. Consequently, reef animals must be adapted for change if they are to persist and spread to occupy suitable environments. It is one thing to note that reef animals are adapted to withstand change; this should be obvious to all but the dimmest light bulb in the package. It is quite another thing, however, to also note, as I will do in this article, that not only are they adapted for change, they actually NEED change.
Daily EnLIGHTenment
It is worthwhile to examine the types of change that are most commonly encountered on coral reefs. To aquarists living in the temperate regions, the most obvious of these changes is probably the regular diurnal cycle of light and dark. It is a trite statement to say that numerous activities and behaviors are regulated by changes in light intensity; yet, it IS worth reiterating that statement. Numerous activities are regulated by changes in light intensity; including feeding, photosynthesis, and virtually all activities of animals such as crabs, shrimp, fishes and aquarists whose sensory input is dominated by vision. The diurnal rhythm of illumination is probably THE single aspect of environmental change that aquarists deal with in a reasonable manner. As such, I really don’t need to deal with it further in this essay.
There are some hidden aspects of these diurnal change on reefs that most hobbyists can’t, don’t, or don’t want to accept and deal with. These aspects are the fact that day length varies continuously throughout the year. The effect of this is more pronounced the closer one gets to the polar areas, but it also occurs in the tropics. In the tropics, another factor that may be as important as the actual magnitude of the change is the position of the sun relative to the zenith. To an equatorial observer, the apparent position of the sun in the sky will vary from 23.5ºN to 23.5ºS of the celestial equator. Changing this factor changes the direction of light impingement and all things that are dependent upon that property of illumination such as shadows, shadow intensity, and amount of total direct illumination. In a very real sense, then, the illumination of aquaria from a light source that does not move with the seasons may be yet another unresolved factor in the husbandry of reef animals.
Time and Tide
“Time and tide wait for no man.”
Geoffrey Chaucer
They don’t wait for reef animals either. The upper edge of a coral reef is absolutely limited by the height of the water over it, so that the reef ends somewhere below the highest water line. In general, only those organisms in the shallowest areas are ever exposed. These shallow areas, those between the highest and lowest tides, are in a zone called, with some uncharacteristic clarity for a biologically used term, “the intertidal zone.” The intertidal zones are bounded by the highest and lowest water levels found in a given region. How wide, or how deep, the intertidal zone is varies with the region. Although the gravitational forces of the moon and sun acting on both the Earth and the water on it generate the tides, the actual magnitude of the tides tends to depend a lot on the local geography. Where the tides push water into and out of triangular- or funnel-shaped embayments the tidal range may be quite large. The highest tides are found in the Bay of Fundy on Canada’s east coast, but other extremely high tidal fluxes are found in the upper reaches of Cook Inlet, near Anchorage, Alaska, and near the head of the Gulf of California. In these regions, the highest and the lowest tides may be separated by more than 10 m (33 ft). In others where the basin doesn’t confine the water mass, such as most coral reef areas, however, the tidal height may fluctuates less than a meter (3.3 ft) over the course of a year. Weather conditions may also influence the tides, winds may blow waters toward or away from the shores either increasing extreme tides, or dampening them out altogether if they blow water in directions contrary to tidal flow. Likewise, high barometric pressure can push water out of an ocean basin lowering the height of tide, while low barometric pressure can allow water to flow into a basin increasing tidal height. The extremely low barometric pressure seen in cyclonic storms, such as hurricanes, contributes to the “storm surge” that is often more devastating that the storms’ winds. However, for reef organisms, possibly more important than the actual exposure of shallow water corals during the tidal cycle, is the movement of water that the tides generate.
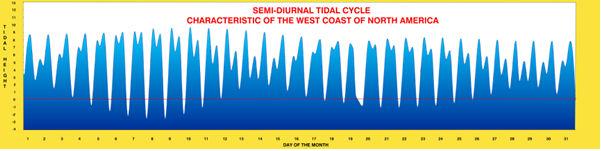
Figure 1. An example of the variation through the month of tidal height in feet related to a datum (= zero level) of Mean Lower Low Water. A semi-diurnal tidal cycle has two unequal high tides and two equal low tides during an average day. Many coral reef areas have a diurnal tidal cycle where both highs and both lows are roughly equivalent to each other. Other areas may have a tidal cycle with one high and one low tide per day. In all cases, however, organisms can adapt to, and use, the tidal cycle as a trigger for essential behaviors.
Water flow in and around coral reefs is due generally to two types of currents that differ in their mode of generation. As it would be trite to say, “water movement is water movement;” to an organism, it might seem trivial to differentiate between the types of water movement; after all, in either case, the medium moves and as long as that movement is within certain specific extremes, the organism can withstand it and survive. However, from the aspect of a biologist or a reef aquarist, it is definitely necessary to discriminate between the types of water flow. Currents of the first type are wind driven oceanic currents. Although there may be some significant variations with the season, these currents tend to be more-or-less steady, in both direction and magnitude. Oceanic currents are vital to coral reefs as they bring to the reef the planktonic food that feeds many of the animals on it. A more variable subset of the wind driven currents would be those generated by storms, in particular the large cyclonic storms, such as hurricanes and typhoons, common in the tropics. Most of these currents are inconsequential, except inasmuch as the worst of them may significantly reorganize the geography of the reef and in the process, they may contribute to the spread of those animals such as shallow-water acroporids that depend upon fragmentation for asexual reproduction. Nonetheless, as far as animals are concerned, a major distinction between these two types of currents is that the former are absolutely periodic and predictable from day to day, and the latter, while “expected” over the lifetime of the organism are not predictable.
The second current category includes those generated by tidal influences. Tides are essentially long oceanic waves of low amplitude and long period generated by the interactions of the gravitational pulls of the moon, sun, and Earth. Waves, as long as they don’t impinge on a surface, generate no net water flow; what sloshes forward also sloshes back. However in shallow basins, where the water moved by these waves encounters the frictional drag of the ocean bottom, all waves including tides, may result in a lot of localized water movement. To the point of this essay, however, such tidally-generated currents have a specific tidal periodicity, changing both magnitude and direction up to four times per day. Interestingly, due to astronomical factors, the tidal pattern is not on a strictly daily scale. Although the earth rotates on its axis once every twenty-four hours, the time for the combined motion of the Earth’s axial rotation and the lunar orbital velocity to put the moon at approximately the same place in the sky on subsequent days is about 24 hours and 50 minutes. Consequently, the corresponding tides are about 50 minutes later each day. The tidal pattern is a consistent and highly predictable one that repeats every 18.6 years. Although the magnitude and directional flows are variable from place to place and, particularly, from ocean basin to ocean basin, they are very predictable in local habitats. This local predictability has significant consequences as natural selection can, and has, attuned the organisms in most places to take advantage of it.
The Periodicity and Constancy Of Change
One of the things, then, that characterizes reef environments is change, and change with a predictable periodicity. If an organism can sense this change, it has the major important consequence of making the world predictable. The question then becomes, “Do organisms seem to be able to predict changes in their environment?” The answer is, undoubtedly and unequivocally, “Yes!” Probably the most common examples of this predictive ability, however, come from temperate marine environments rather than reefs. The reason for the predominance of temperate examples (See, for example, MacGinitie and MacGinitie, 1968; Morris, et al., 1980; Kozloff, 1983; Shimek, 1987; Ruppert and Fox, 1988) is two fold. First, most marine research has been done in temperate areas, and the work that has been there is better known than is the work done in reef environments. Secondly and perhaps more importantly, because of the wider range of swings in both daylight and temperature in temperate areas changes in animal behaviors are easier to document and correlate with the environmental changes. In all temperate marine areas that have been investigated, the working rule is that both generalized and specific behavior patterns are correlated with environmental changes, such as those due to tidal, temperature, and illumination variables.
The filter feeding sea cucumbers of the cool waters of the northern Puget Sound region provide a good example of a series of generalized behaviors that are apparently triggered by environmental cues. There are several different and very common sea cucumbers found in this region including five or more species each of Cucumaria and Pentamera, as well as other species in several other genera such as Eupentacta. These animals are commonly found extended and feeding from late February to late September. During this period, the region’s waters are rich with plankton. Snowmelt and rain generate nutrient-laden runoff, and the annual plankton bloom follows this and is continually fueled by it. The water can be as murky as thick soup during the rich spring and summer plankton blooms, and the cukes take full advantage of this abundant supply of food. There is a risk to feeding, however, as feeding activities increase the cucumber’s exposure to predators. By late summer, however, the bloom tapers off. Shortly thereafter all of these animals cease feeding and disappear into hiding in burrows and under rocks; presumably the benefits gathered by feeding during this period do not outweigh the risks generated by feeding behavior. The reduction in food in the water, resulting in a drop in the amount of food eaten per feeding period may be the proximate cue for this behavioral change, but the cue may also be the slight drop in water temperature that occurs in the fall. The water temperature in the Puget Sound region is far LESS variable than in most coral reef areas, but during the fall it slowly drops a couple of degrees Celsius. Whatever the cues, the sea cucumbers appear to “vanish” from the areas. They withdraw into burrows or into the sediments or under the rocks and become non-feeding and quiescent. They remain out of sight and out of mind until late January or early February. By this time, the amount of daylight has increased significantly from the low of the winter solstice. The water temperature is starting to rise. The water is still clear, however, as the spring plankton bloom has not yet commenced. The benthic environment becomes covered with diatoms where it was clean of them in the dead of winter, and all of a sudden, within a week or two, sea cucumbers appear and inflate into their feeding postures. They are not responding to the presence of food, there really isn’t any more during this period than there was a few weeks before. Temperature and light, however, have changed. A few weeks after this the spring plankton bloom commences and the animals can really start feeding. During this period, they rapidly gain weight and produce gametes. Then on the first sunny day following the spring equinox, on an outgoing or ebbing tide, in the afternoon they spawn. Most of the populations will spawn during this first blast of reproduction, but some animals will also spawn over the next week or so.
Figure 2. The top picture was taken in September, the lower one in November at the same locality in northern Puget Sound illustrating seasonal differences in feeding behavior of sea cucumbers discussed in the text. Note the orange (Cucumaria miniata) and red (Psolus chitonoides) feeding tentacles are visible in the top image and not the lower one. In the lower image, the cucumbers are not feeding. The Cucumaria are withdrawn into burrows and are not visible. The Psolus are visible when not feeding; one is present in the lower image (the orange structure above the snail to the lower right).
Figure 3. Seasonal feeding differences in sea cucumbers are found in soft sediment areas as well in hard substrates. The top picture was taken in July, the lower one in December at the same locality in northern Puget Sound. Note the abundance of white cucumbers (Pentamera populifera) visible in the top image and not the lower one. The cukes are buried under the sediment in the lower image. The scallops are Patinopecten caurinus and are about 8 inches in diameter.
In such habitats, the animals appear to respond to the cues of increasing day length and/or temperature as the signal to begin to feed. Subsequent to that, they appear to use several cues: 1) the equinox, manifested as equally long periods of light and dark in a day, 2) the currents generated by an outgoing tide, and 3) the bright light of a sunny day (which may boost the temperature a bit, as well, as the signals to spawn. And spawn they do… These animals may be very abundant; Cucumaria miniata, a large animal up to 30 cm (1 foot) long and 2 cm (0.8 inch) in diameter, commonly reaches densities of 150 animals per square meter. In some areas, the densities of some species of Pentamera are in excess of 10,000 animals per square meter. When these animals spawn, enough of the large greenish eggs of Cucumaria are released to change the color of moderate sized bays (those about a mile long and wide) from blue to green. All of these events appear to be triggered by environmental changes.
Figure 4. Mass spawning of Cucumaria miniata occurs shortly after the spring equinox while the tide is ebbing on a sunny afternoon. Here a female is releasing some of her eggs.
Reef Coral “Jollies”
Similar things happen on reefs. Until as recently as about twenty-five years ago, no one had ever seen or documented spawning in nature for a reef coral. Rather by happenstance, the first examples of coral spawning were noted by some divers to be nocturnal and synchronous within many species. This type of pattern had been previously seen in other types of animals in temperate regions, and it was realized that what was occurring with the corals was a synchronous spawning event triggered by some environmental cue. In the corals, as with many temperate animals, the spawning is synchronized with the lunar cycle. However, that synchronization doesn’t imply that the cycle is triggered directly by changes in the phase of the moon. In fact, such direct synchronization is rather unlikely. In this type of situation, the signals that trigger spawning are likely to be derived from the lunar cycle, but are unlikely to have any direct relationship to moonlight. The reason for this may be obvious, if the spawning were to be triggered by moonlight alone, a series of stormy nights could seriously disrupt spawning and may even delay the spawning time well past what would be necessary for the survival of the larvae that would result from the spawning event. Moonlight may be one of the cues, but other cues would certainly also be important. The other primary variables linked to lunar periodicity are, of course, the tides and day length.
As corals have no eyes the question might arise as how they are able to measure and assess changes in day or night length. It turns out that in many animals, the synthesis of numerous chemicals is dependent upon light. In humans, for example, vitamin D synthesis is dependent upon the action of ultraviolet light in outer layers of the skin. Similar types of chemical formation can trigger the production of specific hormones in many invertebrates. Often these same chemicals are broken down in darkness. Even the slight, but regular, changes in day length or illumination, such as those that occur in the tropics, can trigger regular patterns in the concentration of a chemical that might be used as triggers for gonad maturation or spawning. For example, after the summer solstice, a slight but progressive lowering of the rate of production of a given photochemical might result in the decrease of an inhibitory material. When the level of the inhibitory material falls below a given threshold, the inhibition ceases; with the cessation of the inhibition might come the initiation of gamete production. Changes in the levels of another chemical, perhaps one made in response to the buffeting of the animals’ bodies in strong tidal currents, could subsequently result in spawning but only if the gonads were “ripe.” Such cybernetic chemical arrays utilizing the interactions of both inhibiting and stimulating chemicals are quite common in many animals. These interactions are often quite complex because they may result from the interactions of several different types of chemicals, all of which may be termed as “hormones.” Under the action of natural selection, the synergy of these chemicals could be fine tuned to give an extremely precise response: the synchronous spawning of a large number of corals of different species. Of course, all the chemicals have to do is to get the animal “primed” for spawning. The final trigger could be the reception of chemical released into the water by any other spawning individual. In other words, once the population is ready to spawn, it would only take the spawning of one animal to trigger all downstream animals to spawn right along with it.
Aquarium Concerns
The deteriorating state of coral reefs (see here, and here) means sooner or later, and mostly likely sooner than later, aquarists will either need to be propagating the animals they are maintaining or they won’t have animals to maintain. Although the techniques of asexual propagation are well known for some animals, these techniques produce only few clones of the original animal. They introduce no new varieties, and because of the traumatic nature of the fragmentation, they are limited in the number of animals that can be produced at any one time. Additionally, cloning by fragmentation in many marine animals is impossible.
If aquarists were familiar with the events necessary for the triggering of sexual reproduction, large numbers of offspring, all new individuals, could be produced at any one time. This would lead both to large numbers of the animals and new color and morphological varieties. By selective manipulation of the appropriate parameters, the spawning event would not be haphazard or unexpected. The few spawning events that have occurred in aquaria have been, without exception, unplanned and unexpected, and most have resulted in problems. That need not be the case, as there is no reason why reef tanks cannot be set up with current and lighting regimes that mimic natural conditions so effectively that spawning times will be known to the nearest half hour. Animals in nature are tuned into the variability of their environment. As hobbyists, we should not only be providing variation to our animals in a natural pattern, we should be planning for the natural outcome of that variation and using it to further our hobby.
References Cited:
Kozloff, E. N. 1983. Seashore Life of the Northern Pacific Coast. An illustrated guide to Northern California, Oregon, Washington, and British Columbia. University of Washington Press. Seattle. 370 pp.
Morris, R. H., D. P. Abbott and E. C. Haderlie. 1980. Intertidal invertebrates of California. Stanford University Press. Stanford. 690 pp.
MacGinitie, G. E. and N. MacGinitie. 1968. Natural history of marine animals. McGraw-Hill Book Co. New York. 523 pp.
Ruppert, E. E. and R. S. Fox. 1988. Seashore Animals of the Southeast. University of Southern Carolina Press. Columbia, S. C. 429 pp.
Shimek, R.L. 1987. Sex among the sessile: With the onset of spring in cool northern Pacific waters, even sea cucumbers bestir themselves. Natural History 96: 60-63.
Coral Spawning References (This Is A Short List, There Are A Lot More). These references are generally available in libraries and provide many of the appropriate data necessary to understand spawning in the animals discussed:
Achituv, Y. and Y. Benayahu. 1990. Polyp dimorphism and functional, sequential hermaphroditism in the soft coral Heteroxenia fuscescens (Octocorallia). Marine Ecology Progress Series. 64:263-269.
Arai, T., M. Kato, A. Heyward, Y. Ikeda, T. Iizuka and T. Maruyama. 1993. Lipid composition of positively buoyant eggs of reef-building corals. Coral Reefs. 12:71-75.
Babcock, R. C. 1984. Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef province. Coral Reefs. 2:187-195.
Babcock, R. C. and A. J. Heyward. 1986. Larval development of certain gamete-spawning scleractinian corals. Coral Reefs. 5:111-116.
Babcock, R. C., G. D. Bull, P. L. Harrison, A. J. Heyward, J. K. Oliver, C. C. Wallace and B. L. Willis. 1986. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Marine Biology (Berlin). 90:379-394.
Beauchamp, K. A. 1993. Gametogenesis, brooding and planulation in laboratory populations of a temperate scleractinian coral Balanophyllia elegans maintained under contrasting photoperiod regimes. Invertebrate Reproduction and Development. 23:171-182.
Benayahu, Y. and Y. Loya. 1984. Life history studies on the Red Sea soft coral Xenia macrospiculata Gohar,. 1940. I. Annual dynamics of gonadal development. Biological Bulletin (Woods Hole). 166:32-43.
Benayahu, Y. and Y. Loya. 1984. Life history studies on the Red Sea soft coral Xenia macrospiculata Gohar,. 1940. II. Planulae shedding and post larval development. Biological Bulletin (Woods Hole). 166:44-53.
Benayahu, Y. and Y. Loya. 1986. Sexual reproduction of a soft coral: Synchronous and brief annual spawning of Sarcophyton glaucum. Biological Bulletin (Woods Hole). 170:32-42.
Chadwick-Furman, N. E. and M. Spiegel. 2000. Abundance and clonal replication in the tropical corallimorpharian Rhodactis rhodostoma. Invertebrate Biology. 119:351-360.
Chadwick-Furman, N. E., M. Spiegel and I. Nir. 2000. Sexual reproduction in the tropical corallimorpharian Rhodactis rhodostoma. Invertebrate Biology. 119:361-369.
Chiappone, M. and K. M. Sullivan. 1996. Distribution, abundance and species composition of juvenile scleractinian corals in the Florida reef tract. Bulletin of Marine Science. 58:555-569.
Delvoye, L. 1988. Gametogenesis and gametogenic cycles in Agaricia agaricites (L.) and Agaricia humilis Verrill and notes on gametogenesis in Madracis mirabilis (Duchassaing & Michelotti) (Scleractinia). Uitgaven Natuurwetenschappelijke Studiekring Voor Suriname En De Nederlandse. Antillen:101-134.
Fabricius, K. E. and J. Metzner. 2004. Scleractinian walls of mouths: Predation on coral larvae. Coral Reefs. 23:245-248.
Fadlallah, Y. H. 1982. The reproductive biology of three species of corals from central California. Dissertation Abstracts International B Sciences and Engineering. 43:948.
Fadlallah, Y. H. 1996. Synchronous spawning of Acropora clathrata coral colonies from the western Arabian Gulf (Saudi Arabia). Bulletin of Marine Science. 59:209-216.
Fan, T. Y. and C. F. Dai. 1995. Reproductive ecology of the scleractinian coral Echinopora lamellosa in northern and southern Taiwan. Marine Biology (Berlin). 123:565-572.
Fan, T. Y. and C. F. Dai. 1999. Reproductive plasticity in the reef coral Echinopora lamellosa. Marine Ecology Progress Series. 190:297-301.
Fautin, D. G. 2002. Reproduction of Cnidaria. Canadian Journal of Zoology. 80:1735-1754.
Fisk, D. A. and V. J. Harriott. 1990. Spatial and temporal variation in coral recruitment on the Great Barrier Reef: implications for dispersal hypotheses. Marine Biology (Berlin). 107:485-490.
Gittings, S. R., G. S. Boland, K. J. P. Deslarzes, C. L. Combs, B. S. Holland and T. J. Bright. 1992. Mass spawning and reproductive viability of reef corals at the East Flower Garden Bank, northwest Gulf of Mexico. Bulletin of Marine Science. 51:420-428.
Glynn, P. W., N. J. Gassman, C. M. Eakin, J. Cortes, D. B. Smith and H. M. Guzman. 1991. Reef coral reproduction in the eastern Pacfic: Costa Rica, Panama, and Galapagos Islands (Ecuador). I. Pocilloporidae. Marine Biology (Berlin). 109:355-368.
Glynn, P. W., S. B. Colley, C. M. Eakin, D. B. Smith, J. Cortes, N. J. Gassman, H. M. Guzman, J. B. Del-Rosario and J. S. Feingold. 1994. Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). 2. Poritidae. Marine Biology (Berlin). 118:191-208.
Glynn, P. W., S. B. Colley, N. J. Gassman, K. Black, J. Cortes and J. L. Mate. 1996. Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). 3. Agariciidae (Pavona gigantea and Gardineroseris planulata). Marine Biology (Berlin). 125:579-601.
Hall, V. R. and T. P. Hughes. 1996. Reproductive strategies of modular organisms: comparative studies of reef-building corals. Ecology (Washington D C). 77:950-963.
Harriott, V. J. 1983. Reproductive ecology of four scleractinian species at Lizard Island, Great Barrier Reef. Coral Reefs. 2:9-18.
Harriott, V. J. 1983. Reproductive seasonality, settlement, and post-settlement mortality of Pocillopora damicornis (Linnaeus), at Lizard Island, Great Barrier Reef. Coral Reefs. 2:151-157.
Harriott, V. J. 1992. Recruitment patterns of scleractinian corals in an isolated sub-tropical reef system. Coral Reefs. 11:215-219.
Harrison, P. L. 1985. Sexual characteristics of scleractinian corals: systematic and evolutionary implications. Proceedings of the Fifth International Coral Reef Symposium. 4:337-342.
Harrison, P. L. and C. C. Wallace. 1990. Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky, Z. Ed. Coral Reefs. Elsevier. Amsterdam. pp. 133-207.
Harrison, P. L., R. C. Babcock, G. D. Bull, J. K. Oliver, C. C. Wallace and B. L. Willis. 1984. Mass spawning in tropical reef corals. Science (Washington D C). 223:1186-1189.
Hayashibara, T., K. Shimoike, T. Kimura, S. Hosaka, A. Heyward, P. Harrison, K. Kudo and M. Omori. 1993. Patterns of coral spawning at Akajima Island, Okinawa, Japan. Marine Ecology Progress Series. 101:253-262.
Heyward, A. J. 1986. Sexual reproduction in five species of the coral Montipora. Hawaii Institute of Marine Biology Technical. Report:170-178.
Heyward, A. J. 1988. Reproductive status of some Guam corals. Micronesica. 21:271-274.
Heyward, A. J. and J. D. Collins. 1985. Growth and Sexual Reproduction in the Scleractinian Coral Montipora digitata (Dana). Australian Journal of Marine and Freshwater Research. 36:441-446.
Heyward, A. J. and R. C. Babcock. 1986. Self- and cross-fertilization in scleractinian corals. Marine Biology (Berlin). 90:191-195.
Heyward, A., K. Yamazato, T. Yeemin and M. Minei. 1987. Sexual reproduction in the corals in Okinawa. Galaxea. 6:331-343.
Holloran, M. K. 1986. The relationship between colony size and larva production in the reef coral Pocillopora damicornis. Hawaii Institute of Marine Biology Technical. Report:167-169.
Hughes, T. P. and J. E. Tanner. 2000. Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology. 81:2250-2263.
Jokiel, P. L., R. Y. Ito and P. M. Liu. 1985. Night irradiance and synchronization of lunar release of planula larvae in the reef coral Pocillopora damicornis. Marine Biology (Berlin). 88:167-174.
Kapela, W. and H. R. Lasker. 1999. Size-dependent reproduction in the Caribbean gorgonian Pseudoplexaura porosa. Marine Biology (Berlin). 135:107-114.
Karlson, R. H. 1981. Reproductive patterns in Zoanthus spp. from Discovery Bay, Jamaica. Proceedings of the Fourth International Coral Reef Symposium. 2:699-704.
Kenyon, J. C. 1992. Sexual reproduction in Hawaiian Acropora. Coral Reefs. 11:37-43.
Kinzie, R. A., III. 1993. Spawning in the reef corals Pocillopora verrucosa and P. eydouxi at Sesoko Island, Okinawa. Galaxea. 11:93-105.
Kojis, B. L. and N. J. Quinn. 1981a. Aspects of sexual reproduction and larval development in the shallow water hermatypic coral, Goniastrea australensis. Bulletin of Marine Science. 31:558-573.
Kojis, B. L. and N. J. Quinn. 1981b. Reproductive strategies in four species of Porites (Scleractinia). Proceedings of the Fourth International Coral Reef Symposium. 2:145-152.
Kojis, B. L. and N. J. Quinn. 1982. Reproductive ecology of two faviid corals (Coelenterata: Scleractinia). Marine Ecology Progress Series. 8:251-255.
Kojis, B. L. and N. J. Quinn. 1984. Seasonal and depth variation in fecundity of Acropora palifera at two reefs in Papua New Guinea. Coral Reefs. 3:165-172.
Kojis, B. L. and N. J. Quinn. 1985. Puberty in Goniastrea favulus age or size limited. Proceedings of the Fifth International Coral Reef Symposium. 4:289-293.
Krupp, D. A. 1983. Sexual reproduction and early development of the solitary coral Fungia scutaria (Anthozoa: Scleractinia). Coral Reefs. 2:159-164.
Lirman, D. 2000. Fragmentation in the branching coral Acropora palmata (Lamarck): Growth, survivorship, and reproduction of colonies and fragments. Journal of Experimental Marine Biology and Ecology. 251:41-57.
Liu, P. J. and T. Y. Fan. 2005. Timing of larval release by the blue coral, Heliopora coerulea, in southern Taiwan. Coral Reefs. 24:30.
Martinelli, F. J. 1986. Time-series analysis of biological data: a case study involving periodicity of coral spawning. Hawaii Institute of Marine Biology Technical. Report:488-501.
Miller, K. J. and C. N. Mundy. 2005. In-situ fertilisation success in the scleractinian coral Goniastrea favulus. Coral Reefs. 24:313-317.
Muller, W. A. and T. Leitz. 2002. Metamorphosis in the Cnidaria. Canadian Journal of Zoology. 80:1755-1771.
Neigel, J. E. and J. C. Avise. 1983. Clonal diversity and population structure in a reef-building coral, Acropora cervicornis: self-recognition analysis and demographic interpretation. Evolution. 37:437-453.
Omori, M., H. Fukami, H. Kobinata and M. Hatta. 2001. Significant drop of fertilization of Acropora corals in 1999: An after-effect of heavy coral bleaching? Limnology and Oceanography. 46:704-706.
Permata, W. D., R. A. Kinzie III and M. Hidaka. 2000. Histological studies on the origin of planulae of the coral Pocillopora damicornis. Marine Ecology Progress Series. 200:191-200.
Reichelt-Brushett, A. J. and P. L. Harrison. 1999. The effect of copper, zinc, and cadmium on fertilization success of gametes from scleractinian reef corals. Marine Pollution Bulletin. 38:182-187.
Richmond, R. H. 1987. Energetic relationships and the biogeographical differences among fecundity, growth and reproduction in the reef coral Pocillopora damicornis. Bulletin of Marine Science. 41:594-604.
Richmond, R. H. 1987. Reproduction and recruitment of corals: comparisons among the Caribbean, the eastern Pacific, the Indo-west Pacific, and the Red Sea. Unesco Reports in Marine Science. 46:239-253.
Richmond, R. H. 1990. Relationships among reproductive mode, biogeographic distribution patterns and evolution in scleractinian corals. Advances in Invertebrate Reproduction. 5:317-322.
Richmond, R. H. and C. L. Hunter. 1990. Reproduction and recruitment among corals: comparisons among the Caribbean, the tropical Pacific, and the Red Sea. Marine Ecology Progress Series. 60:185-203.
Richmond, R. H. and P. L. Jokiel. 1984. Lunar periodicity in larva release in the reef coral Pocillopora damicornis at Enewetak and Hawaii. Bulletin of Marine Science. 34:280-287.
Rinkevich, B. and Y. Loya. 1979. The reproduction of the Red Sea coral Stylophora pistillata. 1. Gonads and planulae. Marine Ecology Progress Series. 1:133-144.
Rinkevich, B. and Y. Loya. 1979. The reproduction of the Red Sea coral Stylophora pistillata. II. Synchronization in breeding and seasonality of planula shedding. Marine Ecology Progress Series. 1:145-152.
Sakai, K. 1998. Effect of colony size, polyp size, and budding mode on egg production in a colonial coral. Biological Bulletin (Woods Hole). 195:319-325.
Sakai, K. and K. Yamazato. 1984. Coral recruitment to artificially denuded natural substrates on an Okinawan reef flat. Galaxea. 3:57-69.
Shlesinger, Y. and Y. Loya. 1985. Coral community reproductive patterns: Red Sea versus the Great Barrier Reef. Science (Washington D C). 228:1333-1335.
Sier, C. J. S. and P. J. W. Olive. 1994. Reproduction and reproductive variability in the coral Pocillopora verrucosa from the Republic of Maldives. Marine Biology (Berlin). 118:713-722.
Simpson, C. J. 1991. Mass spawning of corals on Western Australian reefs and comparisons with the Great Barrier Reef. Journal of the Royal Society of Western Australia. 74:85-91.
Soong, K. 1991. Sexual reproductive patterns of shallow-water reef corals in Panama. Bulletin of Marine Science. 49:832-846.
Steiner, S. C. C. 1995. Spawning in scleractinian corals from SW Puerto Rico (West Indies). Bulletin of Marine Science. 56:899-902.
Stimson, J. S. 1978. Mode and timing of reproduction in some common hermatypic corals of Hawaii and Enewetak. Marine Biology (Berlin). 48:173-184.
Stoddart, J. A. and R. Black. 1985. Cycles of gametogenesis and planulation in the coral Pocillopora damicornis. Marine Ecology Progress Series. 23:153-164.
Stoddart, J. A., R. C. Babcock and A. J. Fleyward. 1988. Self-fertilization and maternal enzymes in the planulae of the coral Goniastrea favulus. Marine Biology (Berlin). 99:489-494.
Szmant, A. M. 1986. Reproductive ecology of Caribbean reef corals. Coral Reefs. 5:43-53.
Szmant, A. M. and N. J. Gassman. 1991. Caribbean Reef Corals. The evolution of reproductive strategies. Oceanus. 34:11-18.
Szmant-Froelich, A., M. Reutter and and L. Riggs. 1985. Sexual reproduction of Favia fragum (Esper): Lunar patterns of gametogenesis, embryogenesis, and planulation in Puerto Rico. Bulletin of Marine Science. 37:880-892.
Szmant-Froelich, A., P. Yevich and and M. E. Q. Pilson. 1980. Gametogenesis and early development of the temperate coral Astrangia danae (Anthozoa: Scleractinia). Biological Bulletin (Woods Hole). 158:257-269.
Tanner, J. E. 1996. Seasonality and lunar periodicity in the reproduction of pocilloporid corals. Coral Reefs. 15:59-66.
Tomascik, T. and F. Sander. 1987. Effects of eutrophication on reef-building corals. 3. Reproduction of the reef-building coral Porites porites. Marine Biology (Berlin). 94:77-94.
Van Moorsel, G. W. N. M. 1983. Reproductive strategies in two closely related stoney corals. Marine Ecology Progress Series. 13:273-283.
Van Veghel, M. L. J. 1994. Reproductive characteristics of the polymorphic Caribbean reef building coral Montastrea annularis. I. Gametogenesis and spawning behavior. Marine Ecology Progress Series. 109:209-219.
Van Veghel, M. L. J. and M. E. H. Kahmann. 1994. Reproductive characteristics of the polymorphic Caribbean reef building coral Montastrea annularis. II. Fecundity and colony structure. Marine Ecology Progress Series. 109:221-227.
Van Veghel, M. L. J. and R. P. M. Bak. 1994. Reproductive characteristics of the polymorphic Caribbean reef building coral Montastrea annularis. III. Reproduction in damaged and regenerating colonies. Marine Ecology Progress Series. 109:229-233.
Villanueva, R. D., H. T. Yap, M. Nemesio and N. E. Montano. 2005. Survivorship of coral juveniles in a fish farm environment. Marine Pollution Bulletin. 51:580-589.
Wallace, C. C. 1985. Reproduction, recruitment and fragmentation in nine sympatric species of the coral genus Acropora. Marine Biology (Berlin). 88:217-233.
Ward, S. 1992. Evidence for broadcast spawning as well as brooding in the scleractinian coral Pocillopora damicornis. Marine Biology (Berlin). 112:641-646.
Westneat, M. W. and J. M. Resing. 1988. Predation on coral spawn by planktivorous fish. Coral Reefs. 7:89-92.
Willis, B. L., R. C. Babcock, P. L. Harrison and C. C. Wallace. 1997. Experimental hybridization and breeding incompatibilities within the mating systems of mass spawning reef corals. Coral Reefs. 16:Suppl.:S53-S65.
Willis, B. L., R. C. Babcock, P. L. Harrison, J. K. Oliver and C. C. Wallace. 1985. Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. Proceedings of the Fifth International Coral Reef Symposium. 4:343-348.
Wilson, J. R. and P. L. Harrison. 1998. Settlement-competency periods of larvae of three species of scleractinian corals. Marine Biology (Berlin). 131:339-345.
Wright, N. 1986. Aspects of reproduction and planula development in the reef coral Cyphastrea ocellina. Hawaii Institute of Marine Biology Technical. Report:179-192.
Wyers, S. C., H. S. Barnes and S. R. Smith. 1991. Spawning of hermatypic corals in Bermuda: a pilot study. Hydrobiologia. 216-217:109-116.
Yamazato, K., M. Sato and and H. Yamashiro. 1981. Reproductive biology of an alcyonacean coral, Lobophytum crassum Marenzeller. Proceedings of the Fourth International Coral Reef Symposium. 2:671-678.
Yeemin, T., S. Nojima and T. Kikuchi. 1990. Sexual reproduction of the scleractinian coral, Montastrea valenciennesi, from a high-latitude coral community, southwest Japan. Publications from the Amakusa Marine Biological Laboratory Kyushu University. 10:105-121.
Zakai, D., O. Levy and N. E. Chadwick-Furman. 2000. Experimental fragmentation reduces sexual reproductive output by the reef-building coral Pocillopora damicornis. Coral Reefs. 19:185-188.