Reef Stability, A Moving Target
By: Ronald L. Shimek Ph.D.
Garbage In… Garbage Out…
One of the more constant concerns of many reef aquarists is keeping their reef “stable.” To this end, large amounts of money and many hours of effort are often spent on all sorts of gadgetry, paraphernalia, and activities all designed or used to keep the aquarium conditions stable. This is done because coral reefs have been described as being in some sort of condition of stability and this statement has entered into both the lexicon of reef aquarists and into their references. This is really, really, unfortunate. The quest for reef stability, much like the quest for the Cup of Christ, is doomed to fail; there is no DaVinci’s Code for the secret of how to succeed. Not the least of the reasons for the inevitable failure in the reef aquarists holy quest is a misunderstanding of the, always undefined, buzzword “stability” in the context of coral reefs. When I was a mere lad, and taking Geometry in high school, we had to do mathematical proofs. For each step in the process, we were supposed to list the mathematical law or postulate that allow that particular step. To save the effort of writing out the same phrase or law repeatedly, our instructor allowed us to abbreviate the phrase with an acronym. It was all very tidy. Not surprisingly, however, occasionally we students, brilliant and excellent though we were, found the appropriate law “missing in action.” My geometry instructor, one Mr. Ludvik Jun, was a man of very finite patience. When the students in his class began sporadically using the acronym MJVC, as a representation of a mathematical law to support a step in their proofs, it didn’t take him long to discover that MJVC meant “Mr. Jun’s Variable Constant.” In other words, it was a “fudge factor” term. It meant precisely whatever we thought it meant at the moment. Mr. Jun was NOT amused! To the point of this essay, however, “stability” is an ecological “variable constant.” It has so many meanings and shades of meanings that unless it is precisely defined in the context of the moment, it is a useless “garbage” term. It is NEVER precisely defined in the reef aquarium literature.
One of the earliest descriptions of coral reefs as having some sort of stability relationship was that of the Odums in their 1955 paper and reiterated by Grassle, in 1973,
“…Coral reef communities are stable ecosystems that have existed in a state of near-equilibrium for millions of years, arising largely as a result of long term and diverse competitive evolutionary adjustments under benign environmental conditions…”
This statement implies that corals are evolutionarily stable, and live under benign or favorable environmental conditions. Please note, that interestingly enough, this statement, although calling the environmental conditions benign, doesn’t say anything about how variable they are.
The concept that coral reefs are evolutionarily stable and live in a environmentally “friendly” environment has slowly morphed over the years into a concept that coral reefs live in an environmentally stable environment without much variation in salinity, temperature, light or any of the other myriad of factors that may be measured. This concept pervades the reef aquarium “literature,” (and I use the word, “literature,” very loosely). As result of this saturation of the literature with ideas of stability and their presumed (but also, interestingly enough, never proven) importance, I think that concerns over all sorts of environmental stability are probably the biggest worries that most reef aquarists are likely to have. And it may be a justified worry, IF the environmental conditions are, indeed, stable. After all, one of the obvious dictums of animal husbandry is that you should maintain animals under the conditions where they do best, and this is, generally, the center of, or the middle point, of the range of conditions in the geographical center of the species range.
IN OTHER WORDS, ORGANISMS DO THEIR BEST WHEN LIVING UNDER THE AVERAGE CONDITIONS OF THE AREA THAT THEY EVOLVED IN.
If those areas have little variation, then the organisms from those areas are generally tolerant of little variation. It should be a relatively simple matter to go to the scientific, oceanographic or climatological, literature and determine what the conditions are on a reef.
Figure 1. A portion of the atoll of the Belau archipelago. The lagoon is to the upper left. The shallow nature of the lagoon indicated by the whitish sand visible under only a few feet of water. The temperature fluctuation in such an environment is quite great.
Average Reef Temperatures
In 1999, Kleypas, and her coworkers, published data on coral reef temperatures (Table 1) (Kleypas, et al, 1999). They examined and summarized published data taken from separate measurements on over 1000 different coral reefs. It is worth remembering that these data were gathered prior to the recent increase in temperatures attributable to global warming and probably reflect more-or-less “normal” conditions for the last couple of centuries. In Table 1, the data in the average column are probably the most pertinent. The average temperature calculated for all 1000+ coral reefs was 81.7°F. Over all reefs, the average annual lowest temperature observed was 76.4°F, and the average annual highest temperature was 86.4°F. Probably the best way that these data could be interpreted would be to say that for most corals and coral reef animals, the best conditions would be between 76°F and 86°F, with the average being about 82°F, and fluctuations of as much as about 5.0°F on either side of that average being acceptable. As I hope to show, in this and my next couple of columns, such variations are not only acceptable for normal and natural coral growth, they are NECESSARY for it.
Coral Reef Temperatures From Kleypas, et al., 1990. All Converted to Degrees Fahrenheit.
| Minimum | Average | Maximum | |
| Averages | 69.8 | 81.7 | 85.1 |
| Minimums | 60.8 | 76.4 | 82.8 |
| Maximums | 76.2 | 86.4 | 93.9 |
Temperature Variations
An area’s average annual temperature does not tell the whole story, of course, as there are always fluctuations around this average. Such fluctuations may vary in magnitude from month-to-month as illustrated in some data from Belize (Table 2). As you read this, it is worth noting that some of the richest coral reefs in the Caribbean have been historically found near Belize, and in these areas average monthly temperatures are generally above 84.1°F (29°C), and the monthly maximum temperature may reach 91.4°F (33°C) (Highsmith, 1979 a, b). These are temperatures slightly cooler than the Indo-Pacific areas of highest coral diversity, but the Belize area is significantly further north. Generally, higher latitude reefs, those further from the equator, have greater temperature extremes than do lower latitudinal, or more equatorially placed, ones.
Surface Water Temperatures for Belize City, Belize, 1964-1971. Data from Highsmith, 1979a.
| Averages | Averages | |||
| Month | °F | °C | °F | °C |
| January | 79.2 | 26.2 | 84.2 | 29.0 |
| February | 80.2 | 26.8 | 84.2 | 29.0 |
| March | 82.4 | 28.0 | 89.6 | 32.0 |
| April | 84.9 | 29.4 | 91.4 | 33.0 |
| May | 86.7 | 30.4 | 91.4 | 33.0 |
| June | 86.2 | 30.1 | 91.4 | 33.0 |
| July | 85.6 | 29.8 | 89.6 | 32.0 |
| August | 87.1 | 30.6 | 89.6 | 32.0 |
| September | 86.2 | 30.1 | 91.4 | 33.0 |
| October | 84.9 | 29.4 | 89.6 | 32.0 |
| November | 81.3 | 27.4 | 87.8 | 31.0 |
| December | 79.9 | 26.6 | 84.2 | 29.0 |
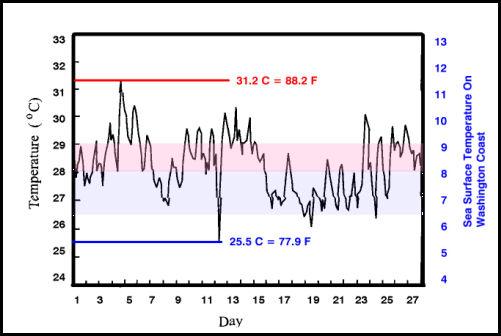
The variation within a single month, at even low latitude reefs can be significantly greater than most aquarists realize. Pulau Pari is an island in Indonesia (5°26′-5°37’S, 106°24′-106°37’E); the variation in the temperature of the shallow reef water in January, 1995 varied over 5.7°C (11.3°F), and the daily extremes were almost as great as the monthly extremes (Figure 2). This island is in a region noted for its coral diversity and these temperatures are typical.
Figure 2. Hourly sea surface temperatures taken 0.5 m below the lowest low water on a shallow coral reef in Pulau Pari, Indonesia, for January 1995 (modified from Fig. 4.3 in Wood, 1999). The red line indicates the maximum temperature, the blue line indicates the minimum temperature, and the pink bar indicates the zone of the average temperature, all values on the left ordinate. For comparison, the values in blue, on the right ordinate, are taken from shallow waters off the Washington coast in the Northeastern Pacific for the same period (values from this NOAA website), the blue band indicates the range of variation seen in that region. Note the range of variation between the highest and the lowest temperature is far greater over the coral reef area than in the offshore area of the Northeastern Pacific. All temperatures in degrees Celsius.
It would be theoretically possible to add data from effectively every coral reef to this short series of examples, but space doesn’t permit it and, in any case, it would be a fruitless exercise in duplication. Coral reef temperatures are variable on tidal, diurnal, weekly, monthly, seasonally, annually, and multiannular time scales and the scope of variation is quite large (Figure 3). Generally, variations of 1.5 ºC to 4.5 ºC (2.7 ºF to 8.1 ºF) are common in equatorial areas, and one-day extremes such as a tidal pool on the Great Barrier Reef of 25.3 ºC to 34.9 ºC (77.5 ºF to 94.8 ºF) are not uncommon (Wood, 1999). Long-term fluctuations in the monthly average sea surface temperature at equatorial localities often fluctuate over a 3ºC to 5 ºC (5.4ºF to 9ºF) range (See Figure 3). When the data for the monthly average fluctuates this much it should be understood that the values for the daily extreme temperatures fluctuate over a much high range. It is worth remembering, that as far as oceanographers and climatologists are concerned, the “sea surface” implied in the measurement of Sea Surface Temperatures (SST) extends to a depth of about 50 m (165 feet) throughout most of the tropics.
Stability With A Grain of Salt
AS WITH THE TEMPERATURE, THE SALINITY OF THE TROPICAL SHALLOW MARINE WATERS IS BY NO MEANS INVARIABLE OR STABLE.
The first factor that needs to be considered is the temperature. Seawater tends to stratify by density, and tropical shallow waters are warmer and, therefore, less dense than the cooler waters under them. The shallow waters float on cooler, denser deep water and they don’t mix with them. The boundary between these water masses is often very sharp. Crossing the boundary will result in a temperature change of several degrees Celsius. Density, salinity, and other factors may also change at the boundary. In any case, this boundary effectively isolates warm tropical shallow waters from the much larger mass of cool water underneath it. Tropical warm waters are seldom more than about 300 m in thickness.
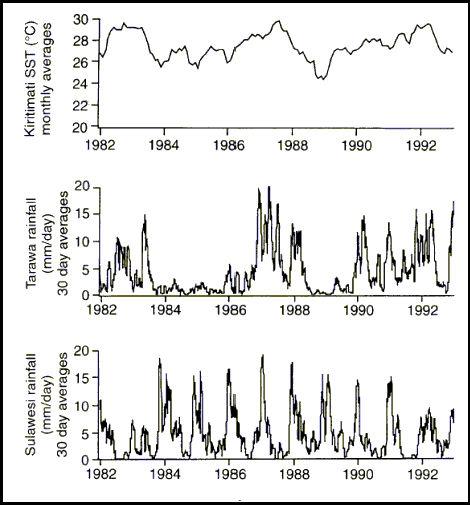
The relative thinness of the tropical warm layer means that it can be subjected to significant changes due to the input of fresh water. This fresh water enters the system either from rainfall or terrestrial run off, and the relative magnitude of each of these factors will vary from locality to locality. The amount of rainfall, even on isolated tropical islands may be a quite large, and quite variable. The monthly average daily rainfall amounts may vary by as much as 20 mm per day. For example, at Tarawa in 1984 through 1985, the average daily rainfall was less than 3 mm per day. In 1987, at the same site, the monthly rainfall averages were never less than 5 mm per day, and as high as 20 mm (0.8 in) per day (Figure 3). Rainfall of this amount can cause significant variation in the salinity of the reef areas.
Outer reefs are generally bathed by oceanic waters, and these are often mixed well and rapidly due to the influence of winds. Consequently, changes in salinity on the outer reefs tend to be transitory, ranging from a few hours to a few days. That isn’t the case in lagoonal areas. Lagoons are often relatively enclosed, and this low frequency of water changing tends to mean that any changes are of long duration. During periods of high rainfall, lagoonal salinities may drop significantly; often to values as low as 30 psu. Because of the relative inefficiency of water movement into and out of lagoons, the salinity may remain low for extended periods; several days to several weeks. Such fluctuation in salinities, and the resultant plankton blooms that may develop if they have been accompanied by nutrient-rich terrestrial runoff, have been hypothesized as one of the causative reasons for the out breaks to of the coral-eating sea star, Acanthaster planci (Birkeland, 1982).
The changes in lagoonal salinities are not all one way. Because lagoons are shallow, and relatively isolated from the surrounding ocean, they also tend to change salinity due to evaporation. If there is little rain, and a lot of evaporation, the salinity may rise. The salinity in some hypersaline lagoons has been measured as high as 42 psu. Needless to say, the variability of lagoonal salinities is a major determining factor in the flora and fauna found in them. Those animals, algae, and plants that can’t tolerate the changes in the salinity will not be found in the lagoons. The flipside of this, though, for aquarists, means that any lagoonal animals in their tanks are likely to be quite tolerant of salinity variations.
Figure 3. Long-term variation in SST (Sea Surface Temperature) and rainfall at some equatorial coral reefs (Modified from Fairbanks, 1997). Note: A SST reading of 27ºC = 80.6ºF. Note, as well, the rainfall is given in 30 day averages, but represents rainfall in mm/day, thus a value of 12.7 mm, means an average of one half inch of rain per day for a month. The regular periodicity of rainfall variation from Sulawesi was due to the annual monsoonal rain pattern.
Rainfall variations of the magnitude shown in Figure 3 will cause significant variations in the sea surface salinities, and such variations are common throughout the coral reef regions of the world. Depending upon the prevailing winds, the land near coral reef areas is often and naturally covered with tropical rain forest, and it should not be surprising that it rains on many reefs, as well.
During periods of little or no rainfall, the seawater in these equatorial regions is generally around 36 psu, but can fluctuate depending on the amounts of terrestrial runoff or deep-water upwelling. When the rains are heavy, the shallow water salinity, down to a depth of 30m (100 feet) or more can be as low as 32 ppt for extended periods. Conversely, during dry periods, there may be significant evaporation within coral lagoons. In these areas the salinity may routinely rise above 37 psu.
In her study of reef temperatures and salinities, Kleypas and her colleagues (1999), found some reefs that had extremely high or low values of temperature or salinity. The values for the Red Sea were particularly high, in terms of both temperature and salinity. The salinity ranged upwards of 38 psu at the southern end, to about 41 psu at the northern end. The average temperature ranged from 88º F to 91.2º F. (31.1º C to 32.8º C). Such temperatures and salinities are at values higher than most aquarists would dare keep their tanks. Nonetheless, those values are the natural ones for the animals of that region.
Stormy Relations
Figure 4. A storm over Chuuk Lagoon. This was just a mild afternoon storm. It altered the next day’s salinity a tad, but by the afternoon of the next the salinity was back to normal.
I would bet real money that for most aquarists, the standard mental image of a coral reef is one of calm seas and blue skies, perhaps with a gentle breeze. Toss in a palm tree on the beach and a Planter’s Punch in one’s hand, some steel guitar music in the background, scantily-clad members of the opposite gender, and the image is one of a version of paradise. Well, yes… but, now, fast forward to when the hurricane hits. Virtually all coral reefs are in parts of the world subject to violent cyclonic storms; these are called hurricanes, typhoons, or cyclones, depending upon where they occur. As we all know, the effects of such storms may be catastrophic and terribly destructive. Nonetheless, they are a periodic and, generally, relatively frequent if unpredictable component of the climate of most coral reef communities. The awesome power of such storms can cause much destruction of reefs, moving large blocks of rock around and simply flushing a lot of animals out to sea. On the other hand, many corals, most especially the acroporids, reproduce mostly by fragmentation and the presence of such storms is a necessary prerequisite for the success and persistence of such species. While we may perceive the advent of a hurricane on the reef as a disaster, such an event may be also interpreted as “Mama Nature’s Frag Fest.” Generally, within a year or two of the awesome destruction of a hurricane, the reef will look fine. Rearranged, perhaps refreshed, but neither abnormal, nor destroyed. As part of the natural environment, these storms have to be tolerated by the organisms in the area. If they weren’t, the organisms would be extinct.
After a period of several million years where there were no reefs, scleractinian corals diversify again about 40 to 50 million years ago. For the first time, scleractinians come to dominate reefs. However, the corals that were found during this period were large massive corals, similar to and including species of Porites. Although branching corals such as Acropora first appeared during this period of diversification, they were rare and remained uncommon. Many modern stony corals, especially acroporids, are generally representatives of evolutionarily very new groups, and are probably less than a couple of million years old (Wood, 1999).
Modern coral reefs are not ancient, but recent phenomena.
“Due to this faunal turnover, late Miocene and Pliocene communities are entirely distinct from early Pleistocene and recent Caribbean reef communities. Although acroporids also appeared in the Eocene, they did not become dominant on reefs until the early Pleistocene (1.8 million years ago). …With this unexplained rise to dominance of branching acroporids in the early Pleistocene, and a corresponding decline in massive, domal corals, coral reef communities with a modern aspect appeared,” (Wood, R. 1999; emphasis and age in years, added).
In point of fact, coral reefs are not evolutionarily stable, but highly dynamic and changeable assemblages.
“…the history of (reef) communities is not simply a quiet and gradual proliferation of lineages… (it) involves dramatic and disconnected episodes, repeated radiations, stagnation, replacement of dominant groups…selective extinction and even worldwide obliteration of entire communities leaving ecological vacuums…”(Newell, 1971).
“Contrary to the previous consensus that emphasized the stability of physical variables at tropical latitudes, the modern, shallow, tropical marine environment is in fact characterized by considerable fluctuations in physical conditions on a daily, seasonal, and annual, and interannual basis….These data have radically altered the view that coral reef communities are stable ecosystems that have existed in a state of near-equilibrium for millions of years, arising largely as a result of long term and diverse competitive evolutionary adjustments under benign environmental conditions (Odum and Odum, 1955; Grassle, 1973). Reefs are clearly not in equilibrium, but present highly dynamic communities that are constantly responding to both constant disturbance, and also to wholly unpredictable catastrophic events.” (Wood, 1999; emphasis added).
In other words, modern coral reefs are not “stable” communities that are the result of long periods of “evolutionary accommodation.” They are highly dynamic and fluctuating associations of organisms containing a mishmash of species in a “free-for-all” fight for existence.
Additionally, coral reefs are in extremely variable environments – on ANY scale you want to measure.
“Shallow water tropical coral reefs occupy changing and often extreme environments…”(Wood, 1999).
“Indeed the constant disturbance and heterogeneity of the coral reef habitat appear to be necessary for the functioning of a healthy coral reef,” (Brown, 1997).
Aquarium Consequences Of These Changes Of Opinion
The viewpoint of researchers concerning the evolutionary and environmental stability of coral reefs has changed dramatically over the last five decades. This is to be expected; coral reef ecology is a relatively new science. As more information is gathered, the previous assumptions and preconceived notions are being shown to be incorrect. This conceptual change has resulted from a tremendous amount of reef research, most of it within the last 15 to 20 years. Scientists now know that not only do corals and coral reef organisms tolerate changing conditions, they may REQUIRE changing conditions for good health.
The implications of this “sea change” in the viewpoint about coral reefs has not percolated through impervious layers of mythology and mythinformation that surround the reef aquarium hobby like a concrete cocoon. When they do – or if they do – aquarists should expect some changes.
They would find there is no reason in the world to keep temperature and salinity in a reef tank stable. In nature, temperature fluctuations of several degrees (Celsius or Fahrenheit) on either side of the average reef temperature of 82º F are normal and of no consequence. They are of no consequence in aquaria as well. The corals “know” this; it is evolutionarily encoded in their genes. Hitherto, most reef aquarists have not been as knowledgeable. Obviously, expensive controllers and chillers are by and large unnecessary, advertising hype to the contrary. Likewise, salinity can vary a bit. Keeping it in the range of 36 to 37 psu is prudent and minimizes the stress on the animals, but some slight variations outside this range are inconsequential.
A Bit Of Foreshadowing…
In my next article, due to be published here in January, 2006, I will discuss how aquarists can use the toleration or, better, the need, for environmental variation in coral reef animals to their advantage in the husbandry of these animals.
References Cited:
Birkeland, C. E. 1982. Terrestrial runoff as a cause of outbreaks of Acanthaster planci (Echinodermata: Asteroidea). Marine Biology (Berlin). 69:175-185.
Brown, B. E. 1997. Adaptations of reef corals to physical environmental stress. Advances in Marine Biology. 31:221-299.
Grassle, J. F. 1973. Variety in coral reef communities. In: Jones, O. A. and R. Endean. Eds. Biology And Geology Of Coral Reefs. Vol. 11. Biology 1: 247-270. 1973.
Fagerstrom, J. A. 1987. The Evolution Of Reef Communities. John Wiley & Sons, Inc. Publishers, New York, NY. 600 pp.
Fairbanks, R. G., M. N. Evans, J. L. Rubenstone, R. A. Mortlock, K. Broad, M. D. Moore, and C. D. Charles. 1997. Evaluating climate indices and their geochemical proxies measured in corals. Coral Reefs. 16, Suppl.: S93-S100
Highsmith, R. C. 1979a. Corals, The Inside Story. Ph. D. Dissertation. Department of Zoology, The University of Washington, Seattle. 322pp.
Highsmith, R. C. 1979b. Coral growth rates and environmental control of density banding. Journal of Experimental Marine Biology and Ecology. 37:105-125.
Kleypas, J. A., J. W. McManus, and L. A. B. Menez. 1999. Environmental Limits to Coral Reef Development: Where Do We Draw The Line? American Zoologist. 39:146- 159.
Newell, N. D. 1971. An outline history of tropical organic reefs. Am. Mus. Novit. 2465, 37 pp.
Odum, H. T. and Odum, E. P. 1955. Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecological Monographs. 25: 291-320.
Wood, R. 1999. Reef Evolution. Oxford University Press. Oxford. 414 pp.